Brandsymbol Releases 5th Edition of the Brand Name Annual FDA Report
A look at the FDA-approved proprietary names and what last year's approvals signal about name safety.
CHARLOTTE, NC, UNITED STATES, January 26, 2026 /EINPresswire.com/ -- Brandsymbol announced the release of the Brand Name Annual FDA Report (5th Edition, 2025), an in-depth review of proprietary names approved by the U.S. Food and Drug Administration in 2025, spanning NMEs, biologics, biosimilars, and vaccines.Designed as an educational, regulatory-grounded industry reference, the report applies Brandsymbol’s SafeMark® Model, FDA Phonetic and Orthographic Computer Analysis (POCA), Word Construction Analysis (WCA), and promotional review principles to examine how approved names perform across safety, similarity, structure, and meaning.
Examples of insights you'd find inside:
- 78% of names had at least one similar pair
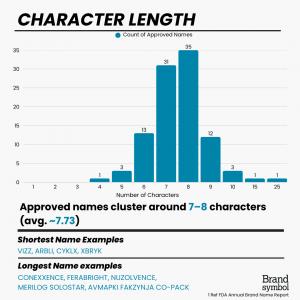
- Average name length: ~7.73 characters, with A, V, B, and E most used
- 23% names hint at the indication
- Biosimilars show a clear naming strategy
The report also explores how promotional review and similarity risk frequently intersect, using nuanced examples such as EXDENSUR, QAMZOVA, and a standout discussion of MIUDELLA, highlighting how subtle associations can influence regulatory outcomes.
The Brand Name Annual Report is now available from Brandsymbol and serves as an essential reference for regulatory, legal, brand, and naming professionals navigating today’s constrained naming landscape.
--> Download the FDA pharmaceutical naming trends report
--> Learn More About Brandsymbol
--> Explore our pharmaceutical naming services
Brandsymbol
+1 704-625-0106
email us here
info@brandsymbol.com
Visit us on social media:
LinkedIn
YouTube
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.